Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.
Contents |
Types of line spectra
Spectral lines are the result of interaction between a quantum system (usually atoms, but sometimes molecules or atomic nuclei) and a single photon. When a photon has about the right amount of energy to allow a change in the energy state of the system (in the case of an atom this is usually an electron changing orbitals), the photon is absorbed. Then it will be spontaneously re-emitted, either in the same frequency as the original or in a cascade, where the sum of the energies of the photons emitted will be equal to the energy of the one absorbed (assuming the system returns to its original state). The direction and polarization of the new photons will, in general, correlate with those of the original photon.
Depending on the type of gas, the photon source and what reaches the detector of the instrument, either an emission line or an absorption line will be produced. Dark lines in a broad spectrum are produced when a cold gas is between a broad spectrum photon source and the detector. In this case a decrease in the intensity of light in the frequency of the incident photon is seen as the photons are absorbed, then reemitted in random directions, which are mostly in directions different from the original one. This results in an absorption line, since the narrow frequency band of light initially traveling toward the detector, has been effectively scattered in other directions. Absorption lines are produced even during reflection from an illuminated cold gas, as after reflection there is still the opportunity for a selective absorption (and re-scatter) between the point of reflection and the detector. In this case the cold gas need not be directly interposed between light source and the detector, but it is required to not act as a significant independent source of light. By contrast, if the detector sees photons emitted directly from a (hot) glowing gas, then the detector often sees photons emitted in a narrow frequency range by quantum emission processes in atoms in the gas, and this results in an emission line.
Spectral lines are highly atom-specific, and can be used to identify the chemical composition of any medium capable of letting light pass through it (typically gas is used). Several elements were discovered by spectroscopic means, such as helium, thallium, and cerium. Spectral lines also depend on the physical conditions of the gas, so they are widely used to determine the chemical composition of stars and other celestial bodies that cannot be analyzed by other means, as well as their physical conditions.
Mechanisms other than atom-photon interaction can produce spectral lines. Depending on the exact physical interaction (with molecules, single particles, etc.) the frequency of the involved photons will vary widely, and lines can be observed across the electromagnetic spectrum, from radio waves to gamma rays.
Nomenclature
Strong spectral lines in the visible part of the spectrum often have a unique Fraunhofer line designation, such as K for a line at 393.366 nm emerging from singly ionized Ca+, though some of the Fraunhofer "lines" are blends of multiple lines from several different species. In other cases the lines are designated according to the level of ionization adding a Roman numeral to the designation of the chemical element, so that Ca+ also has the designation Ca II. Neutral atoms are denoted with the roman number I, singly ionized atoms with II, and so on, so that for example Fe IX (IX, roman 9) represents eight times ionized iron. More detailed designations usually include the line wavelength and may include a multiplet number (for atomic lines) or band designation (for molecular lines). Many spectral lines of atomic hydrogen also have designations within their respective series, such as the Lyman series or Balmer series.
Spectral line broadening and shift
A spectral line extends over a range of frequencies, not a single frequency (i.e., it has a nonzero linewidth). In addition, its center may be shifted from its nominal central wavelength. There are several reasons for this broadening and shift. These reasons may be divided into two broad categories - broadening due to local conditions and broadening due to extended conditions. Broadening due to local conditions is due to effects which hold in a small region around the emitting element, usually small enough to assure local thermodynamic equilibrium. Broadening due to extended conditions may result from changes to the spectral distribution of the radiation as it traverses its path to the observer. It also may result from the combining of radiation from a number of regions which are far from each other.
Broadening due to local effects
- Natural broadening: The uncertainty principle relates the lifetime of an excited state (due to the spontaneous radiative decay or the Auger process) with the uncertainty of its energy. This broadening effect results in an unshifted Lorentzian profile. The natural broadening can be experimentally altered only to the extent that decay rates can be artificially suppressed or enhanced.[1]
- Thermal Doppler broadening: The atoms in a gas which are emitting radiation will have a distribution of velocities. Each photon emitted will be "red"- or "blue"-shifted by the Doppler effect depending on the velocity of the atom relative to the observer. The higher the temperature of the gas, the wider the distribution of velocities in the gas. Since the spectral line is a combination of all of the emitted radiation, the higher the temperature of the gas, the broader will be the spectral line emitted from that gas. This broadening effect is described by a Gaussian profile and there is no associated shift.
- Pressure broadening: the presence of nearby particles will affect the radiation emitted by an individual particle. There are two limiting cases by which this occurs:
-
- Impact pressure broadening: The collision of other particles with the emitting particle interrupts the emission process, and by shortening the characteristic time for the process, increases the uncertainty in the energy emitted (as occurs in natural broadening)[3]. The duration of the collision is much shorter than the lifetime of the emission process. This effect depends on both the density and the temperature of the gas. The broadening effect is described by a Lorentzian profile and there may be an associated shift.
- Quasistatic pressure broadening: The presence of other particles shifts the energy levels in the emitting particle, thereby altering the frequency of the emitted radiation. The duration of the influence is much longer than the lifetime of the emission process. This effect depends on the density of the gas, but is rather insensitive to temperature. The form of the line profile is determined by the functional form of the perturbing force with respect to distance from the perturbing particle. There may also be a shift in the line center. A stable distribution is a general expression for the lineshape resulting from quasistatic pressure broadening [2]
- Pressure broadening may also be classified by the nature of the perturbing force as follows:
- Linear Stark broadening occurs via the linear Stark effect which results from the interaction of an emitter with an electric field, which causes a shift in energy which is linear in the field strength. (
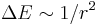 )
) - Resonance broadening occurs when the perturbing particle is of the same type as the emitting particle, which introduces the possibility of an energy exchange process. (
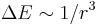 )
) - Quadratic Stark broadening occurs via the quadratic Stark effect which results from the interaction of an emitter with an electric field, which causes a shift in energy which is quadratic in the field strength. (
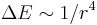 )
) - Van der Waals broadening occurs when the emitting particle is being perturbed by van der Waals forces. For the quasistatic case, a van der Waals profile[note 1] is often useful in describing the profile. The energy shift as a function of distance is given in the wings by e.g. the Lennard-Jones potential (
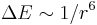 ).
).
- Linear Stark broadening occurs via the linear Stark effect which results from the interaction of an emitter with an electric field, which causes a shift in energy which is linear in the field strength. (
- Inhomogeneous broadening is a general term for broadening because some emitting particles are in a different local environment than others, and therefore emit at a different frequency. This term is used especially for solids, where surfaces, grain boundaries, and stoichiometry variations can create a variety of local environments for a given atom to occupy. In liquids, the effects of inhomogeneous broadening is sometimes reduced by a process called motional narrowing.
Notes:
- ^ "van der Waals profile" appears as lowercase in almost all sources, such as: Statistical mechanics of the liquid surface by Clive Anthony Croxton, 1980, A Wiley-Interscience publication, ISBN 0-471-27663-4, 9780471276630, [1]; and in Journal of technical physics, Volume 36, by Instytut Podstawowych Problemów Techniki (Polska Akademia Nauk), publisher: Państwowe Wydawn. Naukowe., 1995, [2]
Broadening due to non-local effects
Certain types of broadening are the result of conditions over a large region of space rather than simply upon conditions that are local to the emitting particle.
- Opacity broadening: Electromagnetic radiation emitted at a particular point in space can be absorbed as it travels through space. This absorption depends on wavelength. The line is broadened because photons at the line wings have a smaller reabsorption probability than photons at the line center. Indeed, the absorption near line center may be so great as to cause a self reversal in which the intensity at the center of the line is less than in the wings. This process is also sometimes called self-absorption.
- Macroscopic Doppler broadening: Radiation emitted by a moving source is subject to Doppler shift due to a finite line-of-sight velocity projection. If different parts of the emitting body have different velocities (along the line of sight), the resulting line will be broadened, with the line width proportional to the width of the velocity distribution. For example, radiation emitted from a distant rotating body, such as a star, will be broadened due to the line-of-sight variations in velocity on opposite sides of the star. The greater the rate of rotation, the broader the line. Another example is an imploding plasma shell in a Z-pinch.
Combined effects
Each of these mechanisms can act in isolation or in combination with others. Assuming each effect is independent, the observed line profile is a convolution of the line profiles of each mechanism. For example, a combination of the thermal Doppler broadening and the impact pressure broadening yields a Voigt profile.
However, the different line broadening mechanisms are not always independent. For example, the collisional effects and the motional Doppler shifts can act in a coherent manner, resulting under some conditions even in a collisional narrowing, known as the Dicke effect.
See also
- Absorption spectrum
- Atomic spectral line
- Bohr model
- Electron configuration
- Emission spectrum
- Spectrum analysis
- Fraunhofer line
- Hydrogen line
References
- ^ For example, in the following article, decay was suppressed via a microwave cavity, thus reducing the natural broadening: Gabrielse, Gerald; H. Dehmelt (1985). "Observation of Inhibited Spontaneous Emission". Physical Review Letters 55 (1): 67–70. Bibcode 1985PhRvL..55...67G. doi:10.1103/PhysRevLett.55.67. PMID 10031682.
- ^ * Peach, G. (1981). "Theory of the pressure broadening and shift of spectral lines". Advances in Physics 30 (3): 367–474. Bibcode 1981AdPhy..30..367P. doi:10.1080/00018738100101467. http://journalsonline.tandf.co.uk/openurl.asp?genre=article&eissn=1460-6976&volume=30&issue=3&spage=367.